(This article is the basis of an article published at BioProcess International)
Challenge #1
| To develop a system that provides a huge surface area for optimum cell attachment in a reduced volume. The surface should be well suited for cell attachment. The culture area should be enough to produce at least 10 times as many cells as standard devices. The size of the culture device should be such that it can be easily handled by a technician in a laboratory furnished with standard equipment. | 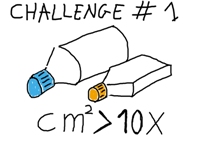 |
Adherent cells need a surface to attach to in order to grow and multiply.
Should you want to increase the number of cultured cells, the obvious strategy is to increase the surface area avaliable for the cells to attach to. This is pretty much the strategy followed in suspension cells culture, where ever larger culture vessels produce larger batches. However, in suspension cells culture, volume is increased after having optimized volumetric productivity. What this means is that before the volume of the culture vessel is increased, the culture process has been optimized to obtain as much product per liter as possible. In the case of adherent cells culture, there exist optimized processes to maximize productivity per square centimeter, but standard culture devices are far from the optimal use of the volume because only a fraction of the volume taken by a culture device is made of a surface available for cell culture.
For example, if we consider a roller bottle, only the inner area of the cylindrical wall is available for cell attachment, and the situation is worse in t-flasks or culture plates, where only the inner surface of the bottom of the device is available for cell growth.
When considering how to solve Challenge #1, some strategies come to mind. The most efficient strategy, as to the amount of surface area, seems to be to introduce particulated supports within the culture device since the amount of surface area you obtain by introducing small particles within a bottle is enormous. Other strategy is to stack culture plates within a device, this way making a better use of the space and avoiding repeated processing steps such as culture medium exchange. And yet another strategy is to roll a large continuous culture membrane or plate within a device, thus providing a continuous membrane that can be fully collonized by the dividing cells.
| Particulated support | Plate stack | Rolled membrane |
 |
 |
 |
Let us briefly consider, one by one, each of these strategies:
Particulated culture supports
|
Using particulated supports for cell attachment provides a huge surface area within the culture vessel. And automation and continuous cultures can be achieved easily since cell culture is carried out in bioreactors long ago used for suspension cells culture. However, there are also some drawbacks associated to the use of particulated supports for adherent cells culture: How do cells from a cell-loaded particle colonize cell-free particles? In traditional culture devices for adherent cells, such as roller bottles, cells simply grow one by another and end up colonizing the whole area available for attachment. That means that a relatively small cell population can colonize a relatively large culture area. However, when using particulated supports for adherent cell culture, every particle need be colonized with some cells from the start of the culture process, since the chances of cells from one particle colonizing another particle are slim. In practice this means that the amount of cells used in the initial seeding must be relatively large. Other considerations include: - Most of the culture vessel must be continuously filled with culture medium, since support particles are mechanically suspended in the medium. Apart from considerations regarding medium usage, gas transfer to growing cells becomes a bottleneck. - Vigorous agitation provokes foaming and particle collisions, exposing the cells to a continuous shear stress. - Agitation of the culture medium with an impeller will lead to fluid speed gradients within the vessel, which in turn will produce a non-homogeneous distribution of the support particles wihtin the culture vessel. The same effect can be expected when shaking the medium using a rocking platform. |
|
Culture plate stack
|
Plate stacks have proven a preferred option for most users pursuing the production of cells (bear in mind the difference between producing cells or using the cells as the factories where another substances are produced). The reasons are plain: traditional culture plates and t-flasks provide a realiable surface to culture adherent cells, and by using a plate stack you eliminate some task repetition. Despite the advantages of plate stacks when compared to t-flasks and culture plates, when considered as a solution to Challenge #1, we encounter some considerations: - Cells multiplying on a plate do not colonize other plates of the stack since each plate of the stack behaves as an independent culture with a common inlet and outlet with the other plates. Therefore, every plate must be seeded at the same cell density in order to obtain similar cell growth on every plate of the stack. - Media usage is not improved as compared to traditional plates since every plate requires the same volume of medium that it would require if it where cultured independently. - Although you eliminate as many step repetitions as plates are in the stack, this is acompanied by a cumbersome handling of the stack, since increasing the number of plates increases proportionally the size of the device. |
|
- As it happens with traditional plates, culture medium must be replaced periodically. This cyclic process step provokes a repeated metabolic stress to the cells and prevents the use of culture stacks on continuous processes.
Rolled membrane:
Roller bottles have proven a sound solution for adherent cell culture. Extensive use of roller bottles has been made both to produce cells and to collect the culture medium where growing cells have excreted target molecules. This success is a consequence of the improved medium usage combined with an increase in culture surface area in an easy to handle device. However, scaling up the use of rolling bottles, although widely used in the industry, does no seem optimum, since the strategy is as plain as using as many bottles as necessary in conditioned laboratories full of rollers that accomodate the huge number of bottles (thousands) to be handled. The alternative of increasing roller bottle diameter is not very sensible (you need a bottle of 1.3 meters in diameter to obtain 10 times as much inner area as in a bottle with a 13 cm diameter) and you can imagine the size of the roller to handle the bottle. However, you can easily introduce a 2.5 meter long rolled membrane within a traditional roller bottle. And since both sides of the membrane will be wetted (and most likely colonized) by the cell culture, you will obtain far more culture area than that of the bottle where the rolled membrane is introduced.
|
However, some considerations must be taken into account: - Liquid flow and distribution in the rolled membrane requires a uniform rotatory movement of the rolled membrane within the bottle. - The massive cell population growing within the device when the rolled membrane is fully colonized demands a continuous medium replacement system and gas exchange to meet metabolic demands of the culture as cells colonize the rolled membrane. |
|
MEETING CHALLENGE #1
As a summary of the findings for each of the solutions considered regarding Challenge #1, we have come up with the following table:
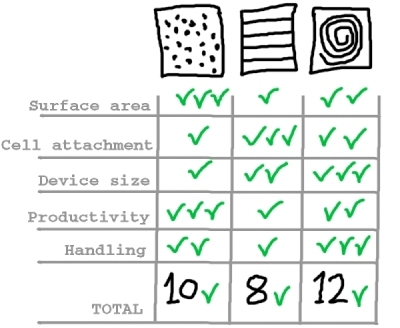
And we conclude that, provided a uniform liquid distribution and automated culture medium and gas replacement system, the best option to meet Challenge #1 is the rolled membrane.
In order to prove our hypothesis, we have built several prototypes. The first one was promising but far from optimum, Finally we have found the rolled membrane shape that provides more than 10 times the surface area of the regular roller bottle in which the rolled membrane is contained. Upon rotation, medium distribution within the device is gentle and uniform throughout the whole membrane.
The liquid flow and distribution wihtin the culture chamber can be appreciated in the following video:
© Copyright 2014 - 2018, The BoB project